Four current and former researchers from the ADDovenom team at the University of Bristol are among the authors of a paper about ADDobodies and ADDomers, published in the journal Structure in January 2024.
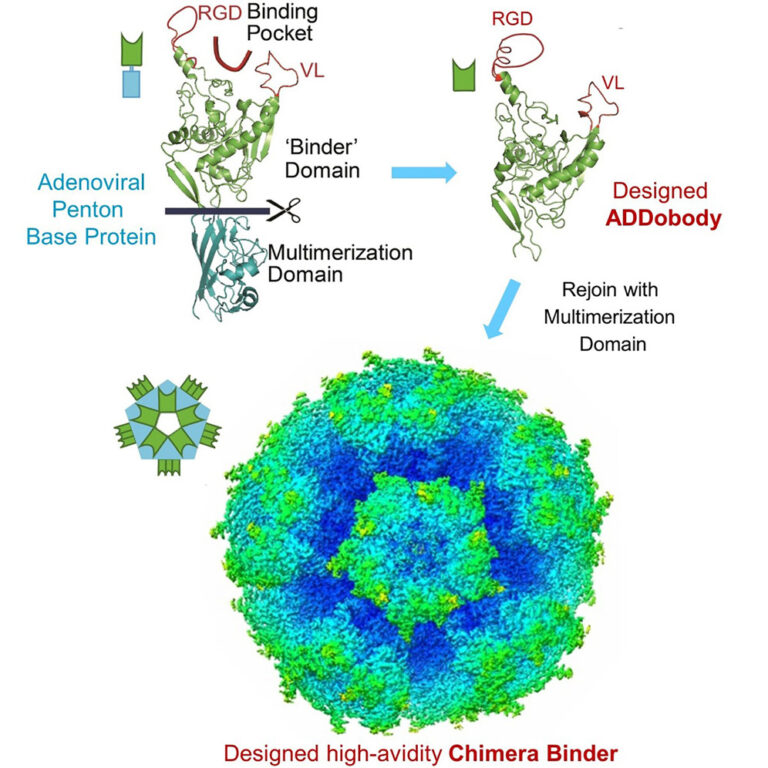
ADDomers (adenovirus-derived nanoparticles) comprise 60 copies of adenovirus penton base protein (PBP). They are thermostable, which means ADDomer-based therapeutics can be stored, transported and deployed without the need for a cold chain. This is of particular importance for the ADDovenom project and the next-generation antivenom treatment it seeks to create because it represents an advantage over conventional IgG-based antivenoms in tropical areas where snakebite envenoming is a significant health, social, and economic burden.
To expand the scope of ADDomers for new applications, the authors engineered ADDobodies validated by high-resolution structure analyses. The ADDobody can be functionalized to interact with a chosen antigen. Fusing the ADDobody to a multimerization domain results in a megadalton-sized protein nanoparticle binding the antigen extremely tightly due to avidity. Ultra-high affinity/avidity is required for detection of toxins or chemicals, and for efficient clearance of toxins from organisms.
- Engineering the ADDobody protein scaffold for generation of high-avidity ADDomer super-binders
Dora Buzas, Huan Sun, Christine Toelzer, Sathish K.N. Yadav, Ufuk Borucu, Gunjan Gautam, Kapil Gupta, Joshua C. Bufton, Julien Capin, Richard B. Sessions, Frederic Garzoni, Imre Berger, Christiane Schaffitzel
Structure (2024), 32, 1–10
https://doi.org/10.1016/j.str.2023.12.010